A Novel Liquid Biopsy for Longitudinal Cancer Monitoring and MRD Detection

Stay Ahead of Cancer Progression

Cancer is dynamic, and timely monitoring is critical for better outcomes.
OncoMonitor® enables oncologists to track disease activity and residual disease in real time — allowing early interventions before clinical relapse becomes visible.
OncoMonitor® TRM: Treatment Response Monitoring and Resistance Tracking
OncoMonitor® MRD: Minimal Residual Disease Detection after therapy
Longitudinal tracking of disease dynamics using blood-based biomarkers
Real-time assessment of treatment efficacy and emerging resistance
Early detection of minimal residual disease (MRD)
Supports prognosis prediction based on CTC and ctDNA trends
AI-powered reporting for rapid, comprehensive insights
How It Works
CTC and ctDNA Quantification
Measures circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) levels from plasma samples.
Longitudinal Monitoring
Regular blood draws allow dynamic assessment of disease burden, therapy response, and relapse risk over time.
Minimal Residual Disease Detection
Trace amounts of ctDNA and CTCs post-treatment signal the presence of residual disease before clinical or radiological signs.
Real-Time AI Reporting
Swift, accurate, and dynamic reports generated through the iCARE™ AI-powered analysis platform.

Applications
For Clinicians & Oncologists
Monitor cancer patients throughout their treatment journey
Adjust therapies based on molecular response or resistance markers
Detect relapse earlier than conventional imaging
For Patients Seeking Personalized Therapy
Minimize invasive procedures with blood-based monitoring
Gain earlier, actionable insights for personalized care adjustments
Why Choose OncoMonitor
Covers both early stage and advanced metastatic disease monitoring
Enables treatment efficacy assessment through longitudinal CTC/ctDNA tracking
Identifies resistance mechanisms and molecular relapse signals
AI-driven, real-time reporting enhances decision-making speed and accuracy

ctDNA Epigenomics Validation Results
| Sensitivity | 90.5% |
| PPV | 95% |
| Specificity | 97.2% |
| NPV | 94.6% |
| Concordance | 94.7% |
| Limit of Detection (LOD) | 0.05 |
Analysis performed on 57 samples including Seraseq® reference standards and healthy individual controls.
Pioneering Precision Monitoring
| Longitudinal Monitoring | Assess disease burden dynamically over time |
| Minimal Residual Disease Detection | Identify occult disease post-therapy |
| Real-Time Results | Swift, AI-powered reporting to support clinical decisions |
| Prognostic Value | Predict outcomes based on molecular response trends |
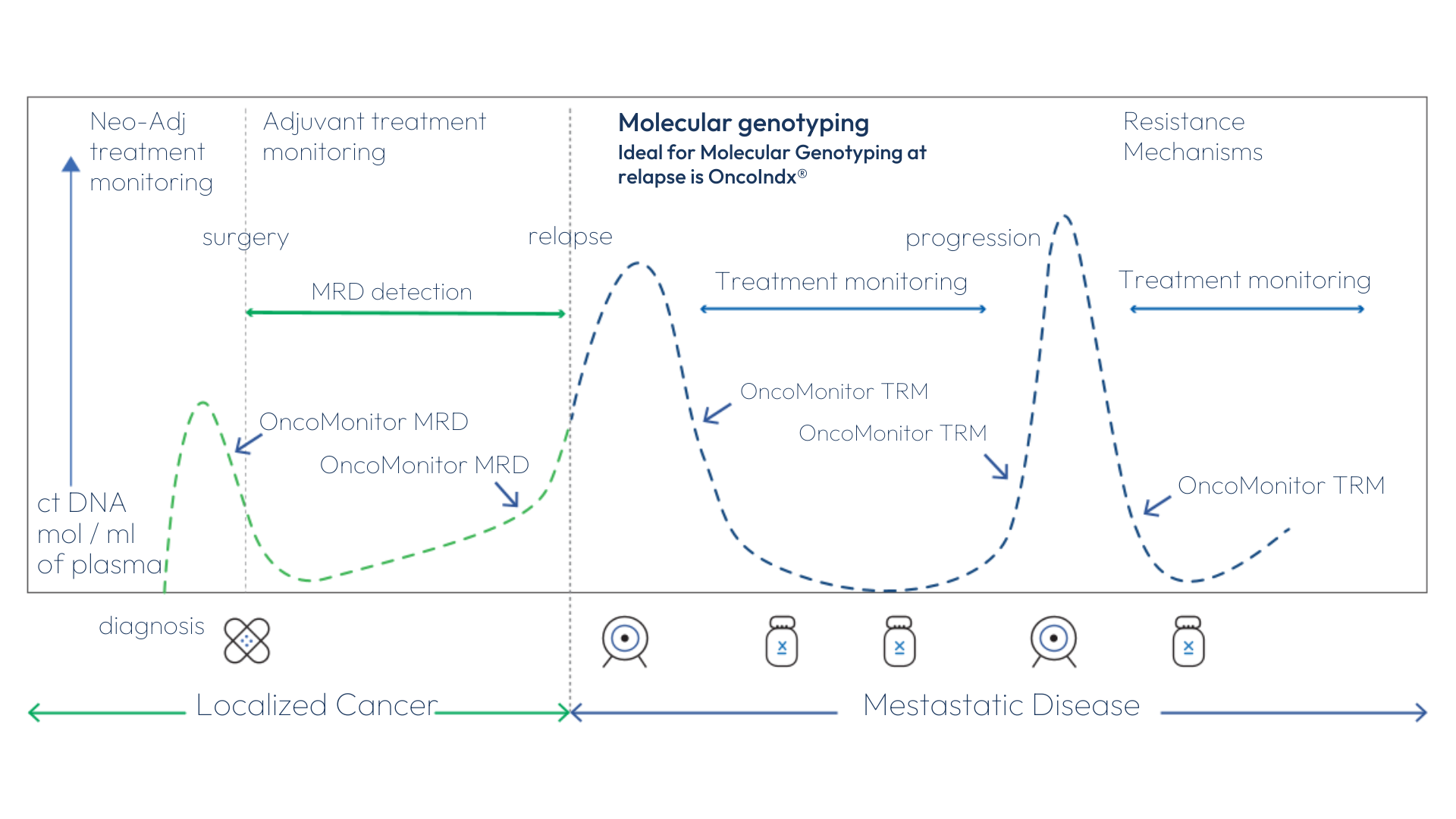
For which cancer phase is OncoMonitor® most applicable?
Applicability Across Cancer Journey: Tracking Disease Progression and Relapse Detection Through Longitudinal Blood-Based Monitoring
Key Actionable Genes Covered (OncoMonitor® TRM)
Lung
EGFR, KRAS, MET, ALK, ROS1, RET, TP53, PIK3CA, HRAS, FGFR1/2/3, ERBB2, NRAS, NTRK1/2/3, MAP2K1, PTEN
Breast
BRCA1, BRCA2, HER2 (ERBB2), PIK3CA, ESR1, PALB2, TP53, FGFR1/2/3
Colorectal
KRAS, NRAS, BRAF, TP53, SMAD4, MLH1, MSH2, MSH6, PMS2, PIK3CA, MET
Melanoma
BRAF, NRAS, KIT, PDGFRA, CTNNB1, HRAS, PIK3CA
Gastrointestinal
KRAS, BRAF, PDGFRA, KIT, MET, SMAD4, MLH1, MSH2, NTRK1/2/3, TP53
Ovary
BRCA1, BRCA2, PALB2, BRAF, KRAS, NRAS, TP53
Bladder
TP53, TSC1, MLH1, MSH2, MSH6, PMS2, NTRK1/2/3