Tissue–Liquid–Normal Match NGS Test for Comprehensive Cancer Profiling
OncoIndx® Prime+ is a next-generation genomic test designed for oncologists managing complex, advanced, or treatment-refractory solid tumors. This powerful platform delivers high-resolution insights through simultaneous analysis of tissue, liquid biopsy, and matched normal DNA—enabling confident therapeutic decisions with unmatched precision.

One Test. Three Matched Inputs. Maximum Clarity.

OncoIndx® Prime+ enables integrated somatic and germline mutation analysis, transcriptomics, and real-time resistance monitoring from a single test.
By combining tumor DNA (tDNA), circulating tumor DNA (ctDNA), and germline DNA, it offers deep visibility into tumor behavior, resistance mechanisms, and actionable targets—supported by the iCare™ AI reporting platform.
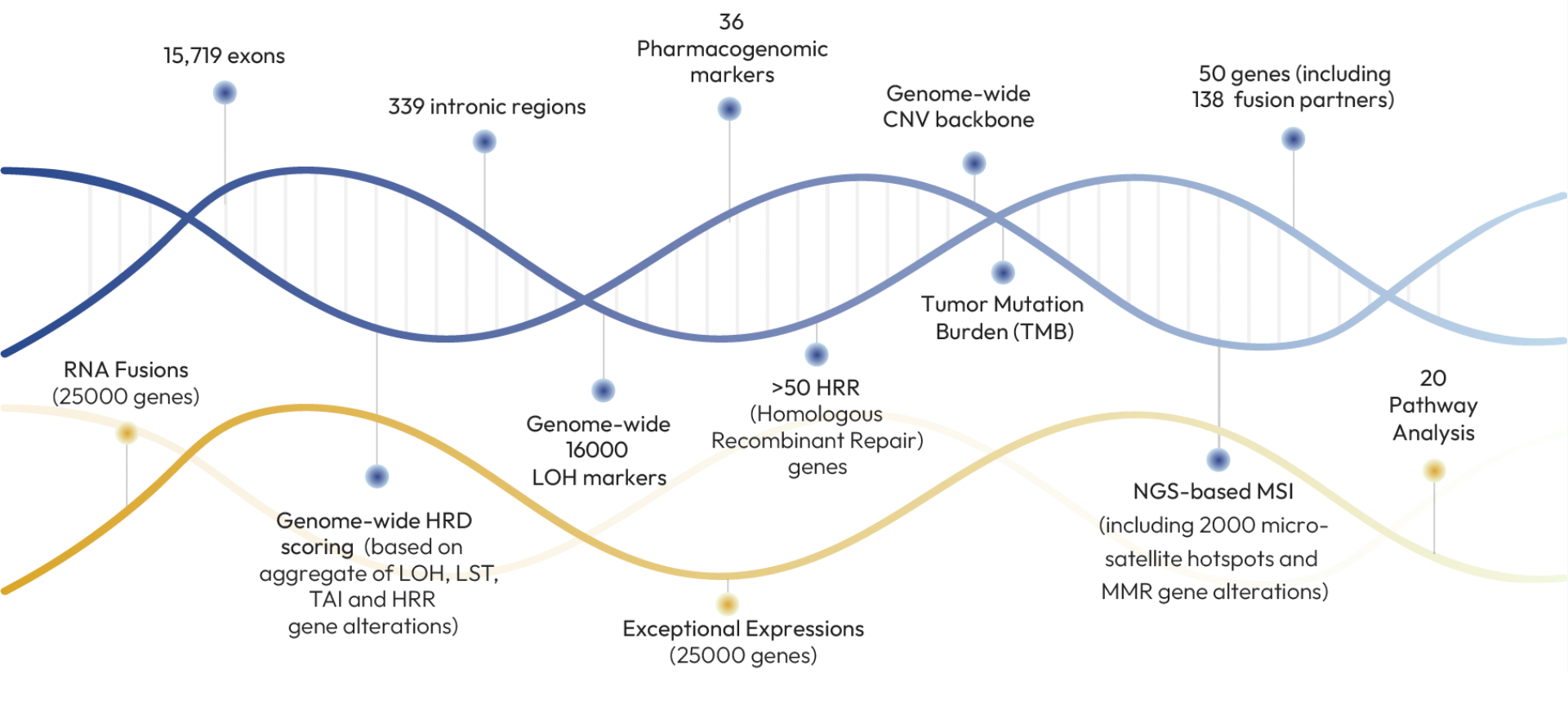
1,000+ gene proprietary panel including 50+ fusion drivers
Tissue, liquid biopsy, and germline matched in a single test
RNA-based fusions, expression analysis, splice variants, and CHIP detection
Genome-wide profiling: TMB, MSI, HRD (LOH, LST, TAI)
36 pharmacogenomics markers for drug response
High accuracy: >99% coverage, 2000x (tissue) / 10000x (liquid) depth & AI-enhanced report with curated, therapy-ready insights
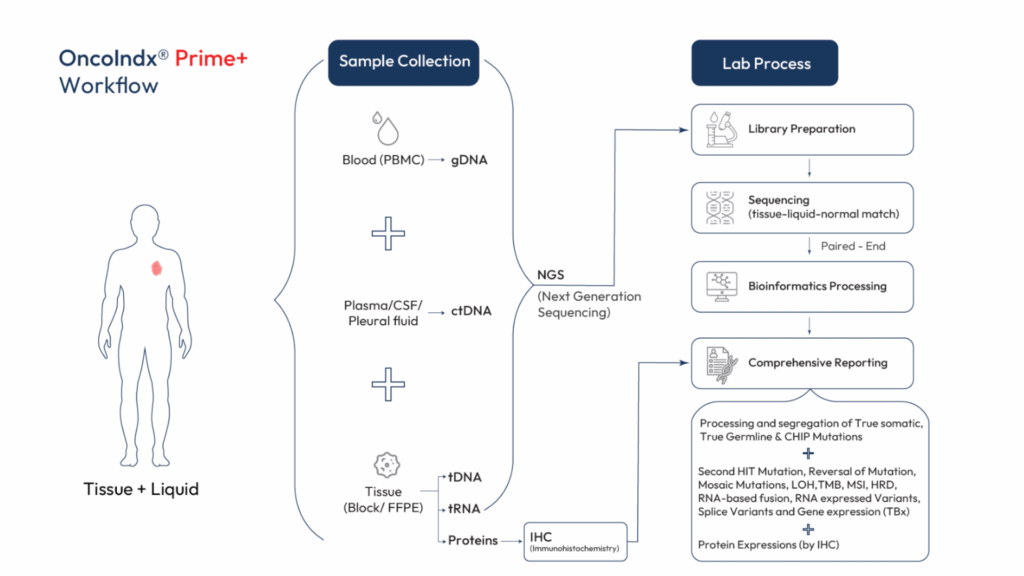
How It Works
Sample Collection
Tissue (FFPE), blood (plasma and buffy coat), or CSF/pleural fluid samples are collected and processed in a CLIA-certified environment.
Library Preparation & Sequencing
NGS is performed using a paired-end approach to sequence tumor, liquid, and matched normal DNA—followed by high-throughput RNA analysis for fusion detection and gene expression.
Bioinformatics Pipeline
Mutations are precisely segmented into true somatic, true germline, CHIP, and mosaic categories using proprietary algorithms.
Comprehensive Report Generation
Final reports are powered by iCare™, featuring fusion detection, actionable variants, pharmacogenomic insights, and therapy recommendations.

Applications
For Oncologists
Resolve complex diagnostic cases and unclear mutational origins
Monitor longitudinal progression with liquid biopsy markers
Identify rare fusions and transcript variants to guide targeted therapies
Avoid confounding germline/CHIP noise with true somatic separation
For Translational Researchers
Integrate CNV, expression, and fusion profiling into one workflow
Perform exploratory research on reversal mutations, second-hit events
Leverage true matched genomic context across tumor types
Why Choose OncoIndx® Prime+?
Combines genomic, transcriptomic, and germline data in one test
Clear distinction between somatic, germline, CHIP, and reversal mutations
Ideal for advanced-stage or recurrent patients with uncertain therapy paths
Validated with >95% sensitivity, specificity, PPV, and NPV across all variant classes
Compatible with both tissue-only, liquid-only, or matched hybrid samples
Specimen & Workflow Overview
| Sample Types | Collection |
|---|---|
| Tissue | FFPE block with ≥20–30% tumor content |
| Liquid Biopsy | Plasma/CSF (ctDNA), PBMC (gDNA), buffy coat |
| Normal DNA | Extracted from blood (gDNA) |
| Input Requirements | >1 ng DNA/RNA |
| Sequencing Platform | Illumina, Paired-End 2×150 bp |
| Depth | 2000x (tissue), 10000x (liquid biopsy) |
| Turnaround Time | 10–14 Days |
Clinical and Technical Validation of OncoIndx® Assay
| Alteration | *PPV | *NPV | Accuracy | Specificity | Sensitivity |
|---|---|---|---|---|---|
| SNVs | 100 | 100 | 100 | 100 | 100 |
| Small INDELs | 100 | 94.03 | 97.40 | 100 | 95.60 |
| CNA | 100 | 100 | 100 | 100 | 100 |
| Fusions | 100 | 96.43 | 98.48 | 100 | 97.44 |
| Sensitivity | *PPV | *NPV | Specificity | Concordance |
|---|---|---|---|---|
| 86.7% | 100% | 100% | 93.6% | 95% |
Note: A total of 165 samples including reference standards, clinical, cross-laboratory and TCGA samples.
*NPV: Negative predictive value. PPV: Positive predictive value.
Designed for oncologists managing complex, advanced and refractory cancer patients.
Advanced Stage Cancers
Including progressive and treatment-resistant tumors
Rare Cancers
Sarcoma, Glioma to identify clinical-trial enrolment or off-label therapy
Cancers of Unknown Primary (CUP)
RNA-based gene expression analysis supports tissue-of-origin identification
Multiple or Dual Malignancies
Distinguish between independent primary tumors and metastatic outcome
Biomarker Profiling
Patient selection for treatment with Immunotherapy / Targeted therapy

What Makes OncoIndx® Prime+ Unique
| Feature | Advantage |
|---|---|
| Tissue–Liquid–Normal Matched Design | Improves precision of somatic vs. germline interpretation |
| RNA-Based Fusion & Expression | Identifies gene rearrangements and splice events missed by DNA alone |
| Genome-Wide HRD Scoring | Combines LOH, TAI, LST, and HRR alterations |
| iCare™ AI Reports | Enables fast, clinician-ready interpretations based on current global guidelines |
| Validated Accuracy | >95% sensitivity, specificity, PPV, and NPV across variant types |
Designed for oncologists managing complex, advanced and refractory cancer patients.